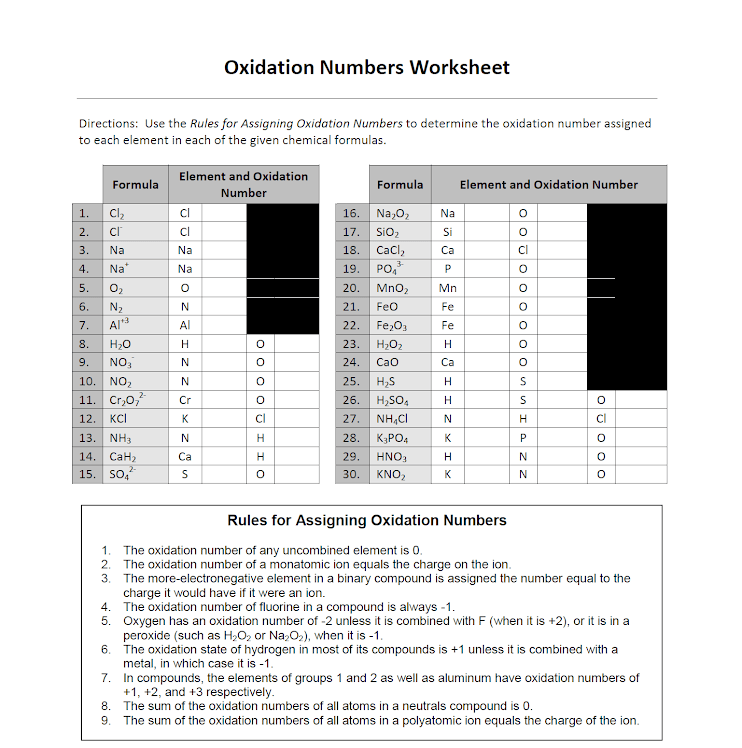
Oxidation Numbers Worksheet Answer Key - The charge on all metals of group 2 of the periodic table is. 2 cr+ + sn4+ → 2 cr3+ + sn2+. The charge on all free elements is zero. The charge on all metals of group 1 of the periodic table is +1 c. The oxidation number of an element in a monatomic ion equals the charge of the ion. A pure element has an oxidation number of 0. Identify the species being oxidised and reduced in each of the following reactions and write their half reactions: Assign oxidation numbersto each of the atoms in the following compounds:
Worksheet Oxidation Numbers Answer Key
The charge on all metals of group 1 of the periodic table is +1 c. A pure element has an oxidation number of 0. The oxidation number of an element in a monatomic ion equals the charge of the ion. Identify the species being oxidised and reduced in each of the following reactions and write their half reactions: Assign oxidation.
Oxidation Number Worksheet
The charge on all metals of group 1 of the periodic table is +1 c. A pure element has an oxidation number of 0. The charge on all free elements is zero. The oxidation number of an element in a monatomic ion equals the charge of the ion. Identify the species being oxidised and reduced in each of the following.
Assigning Oxidation Numbers Worksheet Answers
Identify the species being oxidised and reduced in each of the following reactions and write their half reactions: A pure element has an oxidation number of 0. Assign oxidation numbersto each of the atoms in the following compounds: The oxidation number of an element in a monatomic ion equals the charge of the ion. 2 cr+ + sn4+ → 2.
Assigning Oxidation Numbers Worksheet Answer Key Worksheet Resume
A pure element has an oxidation number of 0. The charge on all free elements is zero. 2 cr+ + sn4+ → 2 cr3+ + sn2+. The charge on all metals of group 1 of the periodic table is +1 c. Assign oxidation numbersto each of the atoms in the following compounds:
Oxidation Reduction Worksheet Answer Key Printable Word Searches
The oxidation number of an element in a monatomic ion equals the charge of the ion. Assign oxidation numbersto each of the atoms in the following compounds: 2 cr+ + sn4+ → 2 cr3+ + sn2+. A pure element has an oxidation number of 0. The charge on all free elements is zero.
Oxidation Reduction Worksheet Answers
2 cr+ + sn4+ → 2 cr3+ + sn2+. A pure element has an oxidation number of 0. The charge on all free elements is zero. Assign oxidation numbersto each of the atoms in the following compounds: The charge on all metals of group 2 of the periodic table is.
Oxidation Numbers Worksheet Thekidsworksheet
2 cr+ + sn4+ → 2 cr3+ + sn2+. A pure element has an oxidation number of 0. The charge on all metals of group 1 of the periodic table is +1 c. The charge on all metals of group 2 of the periodic table is. Identify the species being oxidised and reduced in each of the following reactions and.
Chemistry Balancing Equations Worksheet Answer Key › Athens Mutual
The charge on all free elements is zero. The charge on all metals of group 1 of the periodic table is +1 c. The charge on all metals of group 2 of the periodic table is. Identify the species being oxidised and reduced in each of the following reactions and write their half reactions: A pure element has an oxidation.
Worksheet Oxidation Numbers Answer Key
The charge on all metals of group 1 of the periodic table is +1 c. 2 cr+ + sn4+ → 2 cr3+ + sn2+. A pure element has an oxidation number of 0. The charge on all free elements is zero. Assign oxidation numbersto each of the atoms in the following compounds:
️Determining Oxidation Numbers Worksheet Answers Free Download Gmbar.co
The charge on all metals of group 1 of the periodic table is +1 c. Identify the species being oxidised and reduced in each of the following reactions and write their half reactions: Assign oxidation numbersto each of the atoms in the following compounds: The oxidation number of an element in a monatomic ion equals the charge of the ion..
The charge on all metals of group 1 of the periodic table is +1 c. The oxidation number of an element in a monatomic ion equals the charge of the ion. The charge on all free elements is zero. A pure element has an oxidation number of 0. Assign oxidation numbersto each of the atoms in the following compounds: Identify the species being oxidised and reduced in each of the following reactions and write their half reactions: The charge on all metals of group 2 of the periodic table is. 2 cr+ + sn4+ → 2 cr3+ + sn2+.
The Charge On All Free Elements Is Zero.
Identify the species being oxidised and reduced in each of the following reactions and write their half reactions: The oxidation number of an element in a monatomic ion equals the charge of the ion. A pure element has an oxidation number of 0. The charge on all metals of group 1 of the periodic table is +1 c.
2 Cr+ + Sn4+ → 2 Cr3+ + Sn2+.
Assign oxidation numbersto each of the atoms in the following compounds: The charge on all metals of group 2 of the periodic table is.