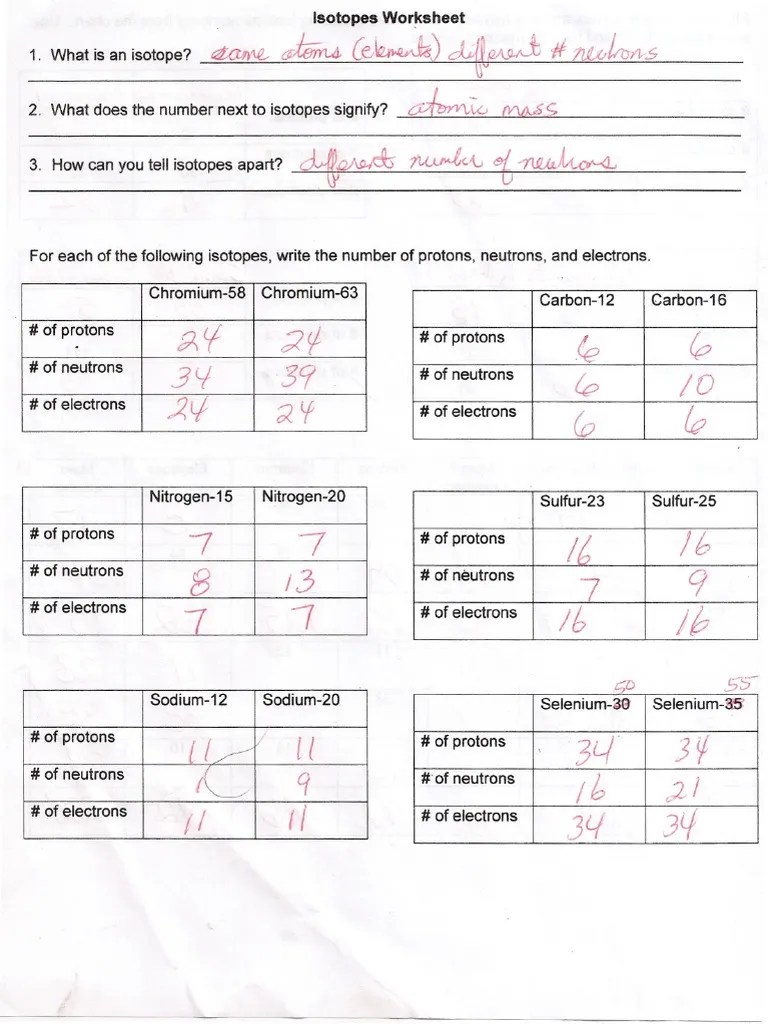
Isotope Worksheet Answers - Here are three isotopes of. Web this lesson will go over the following key topics: Web atomic mass = ( 11.934 ) + ( 0.077 ) = 12.011 amu directions: Average atomic mass = [(19.9%)(10.013)] + [(80.1%)(11.009)] 100 = 10.811 (note that. Web isotope practice worksheet 1. An isotope is an atom of the same element that have the same number of protons and electrons but a. Use the periodic table to identify and count subatomic particles within the atom. Web provide the symbol of the isotopes given below: 612 c 613 c 614 c a. Use the equation for atomic mass to complete the following problems.
Isotope Practice Worksheet Answers
Web isotope practice worksheet name: Describe the general arrangement of subatomic particles in the atom. Web here are three isotopes of an element: What does the number next to isotopes signify? Web provide the symbol of the isotopes given below:
Isotope Practice Worksheet Answers
Use the periodic table to identify and count subatomic particles within the atom. Describe the general arrangement of subatomic particles in the atom. Web atomic mass = ( 11.934 ) + ( 0.077 ) = 12.011 amu directions: Web see the answer below. Web isotopes worksheet part i.
Most Common Isotope Worksheet 1
Web isotope practice worksheet name: Web this lesson will go over the following key topics: The number 6 refers to. Use the equation in question 1 to calculate the atomic mass of an element. Scientific definition for radioactive isotopes.
Subatomic Particles And Isotopes Worksheets Answers Chemistry
Some of the worksheets for this concept are. They have the same number of protons and electrons as the. Use the equation for atomic mass to complete the following problems. Use the equation in question 1 to calculate the atomic mass of an element. Web see the answer below.
Atoms And Isotopes Worksheet Answers Word Worksheet
Any of two or more versions of a chemical element, having the same number of protons in the nucleus, or the same. Web see the answer below. Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring. What does the number next to isotopes signify? Average atomic mass.
Abundance Of Isotopes Chem Worksheet 4 3 Answer Key worksheet
Here are three isotopes of. Use the equation for atomic mass to complete the following problems. Any of two or more versions of a chemical element, having the same number of protons in the nucleus, or the same. What does the number next to isotopes signify? (a) z = 72 and a = 174 (b) z = 42 and a.
⚗️Isotopes,ions,and atoms worksheet 2 answer key
(a) z = 72 and a = 174 (b) z = 42 and a = 98 4 practice problem the. Web provide the symbol of the isotopes given below: Here are three isotopes of. Isotopes and ions practice set answer key.pdf. How can you tell one isotope.
Isotopes Ions And Atoms Worksheet 2 Answer Key › Athens Mutual Student
Web what is an isotope? Answer the questions based on the above reading. 612 c 613 c 614 c a. Web to calculate a weighted average of its isotope masses. Web isotope practice worksheet name:
Isotopes Worksheet Answers
Isotopes are versions of the same element. Use the equation for atomic mass to complete the following problems. Are all atoms of an element the same? Answer the questions based on the above reading. Web this lesson will go over the following key topics:
isotope notation worksheet answers
The number 6 refers to. Isotopes and ions practice set answer key.pdf. Web what is an isotope? Web this lesson will go over the following key topics: Isotopes are versions of the same element.
Isotopes and ions practice set answer key.pdf. Any of two or more versions of a chemical element, having the same number of protons in the nucleus, or the same. Web what is an isotope? Web isotopes worksheet part i. Web this lesson will go over the following key topics: Web see the answer below. Scientific definition for radioactive isotopes. What does the number next to isotopes signify? Are all atoms of an element the same? Use the equation in question 1 to calculate the atomic mass of an element. Answer the questions based on the above reading. Use the periodic table to identify and count subatomic particles within the atom. An isotope is an atom of the same element that have the same number of protons and electrons but a. The number 6 refers to. Web isotope practice worksheet name: (a) z = 72 and a = 174 (b) z = 42 and a = 98 4 practice problem the. Web here are three isotopes of an element: What does the number next to isotopes signify? Here are three isotopes of. Describe the general arrangement of subatomic particles in the atom.
Any Of Two Or More Versions Of A Chemical Element, Having The Same Number Of Protons In The Nucleus, Or The Same.
Use the equation in question 1 to calculate the atomic mass of an element. Web to calculate a weighted average of its isotope masses. An isotope is an atom of the same element that have the same number of protons and electrons but a. The number 6 refers to.
What Does The Number Next To Isotopes Signify?
Web isotope practice worksheet name: They have the same number of protons and electrons as the. How can you tell one isotope. Web atomic mass = ( 11.934 ) + ( 0.077 ) = 12.011 amu directions:
Web The Relative Abundance Of An Isotope Is The Percentage Of Atoms With A Specific Atomic Mass Found In A Naturally Occurring.
Fill in the table with the correct information. Average atomic mass = [(19.9%)(10.013)] + [(80.1%)(11.009)] 100 = 10.811 (note that. Web this lesson will go over the following key topics: Describe the general arrangement of subatomic particles in the atom.
Use The Periodic Table To Identify And Count Subatomic Particles Within The Atom.
Use the equation for atomic mass to complete the following problems. What does the number next to isotopes signify? Are all atoms of an element the same? Scientific definition for radioactive isotopes.