Chemical Reaction Worksheet Answer Key - ____ nabr + ____ ca(oh)2 ___ cabr2 + ____ naoh. Web click the buttons to print each worksheet and associated answer key. All reactants and products require a coefficient of at least one. Web a chemical reaction is a process in which some substances change into different substances. 1) synthesis (slide notes) *worksheet #2: Web identify the oxidizing agent, reducing agent, substance oxidized, and substance reduced in this reaction: Fe (no 3) 3 (aq) + h 2 s. Web types of reactions worksheet balance the following equations and indicate the type of reaction taking place: Preparation for your unit assessment(s) Sii4 + mg → (single replacement) 2al + 3i2 → (synthesis).
Understanding The Different Types Of Chemical Reactions Worksheet
Web predicting products of chemical reactions. Chemical reactions happen when the _________ hook together. Web types of reactions worksheet balance the following equations and indicate the type of reaction taking place: Web for each reaction in part i and part ii record your observations, molecular equation, total ionic equation and net ionic equation. All reactants and products require a coefficient.
Balancing Chemical Equations Worksheet 2 Answer Key Worksheets Samples
Web balance each of the following reactions and identify each type of reaction: You know that you have a balanced chemical equation when the. Preparation for your unit assessment(s) 1) synthesis (slide notes) *worksheet #2: Web this worksheet set includes many detailed problems for students to complete, and also examples for each, involving.1).
10++ Types Of Chemical Reactions Worksheet Answer Key
Web answer key teacher notes on next page! Fe (no 3) 3 (aq) + h 2 s. Web some of the worksheets for this concept are chemical reactions work answer key, identifying chemical reactions work. 1) synthesis (slide notes) *worksheet #2: Web this worksheet set includes many detailed problems for students to complete, and also examples for each, involving.1).
14 Best Images of Chemical Reactions Worksheet Types Chemical
A = red, b = blue, c = green, d = yellow 2. Web types of chemical reactions (*summary handout): Web answer key teacher notes on next page! 1) synthesis (slide notes) *worksheet #2: Write each symbol by each meaning.
Types Of Chemical Reaction Worksheet Ch 7 —
All reactants and products require a coefficient of at least one. Write each symbol by each meaning. Web answer key teacher notes on next page! Web classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a. Web balance the following chemical equation:
Types Of Chemical Reactions Worksheet Answer Key
1) synthesis (slide notes) *worksheet #2: This worksheet is designed to help you predict products. Web predicting products of chemical reactions. Web identify the oxidizing agent, reducing agent, substance oxidized, and substance reduced in this reaction: Web types of reactions worksheet balance the following equations and indicate the type of reaction taking place:
10++ Types Of Chemical Reactions Worksheet Answer Key
Balancing reactions provide the coefficients to balance the. This worksheet is designed to help you predict products. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3. Web balance the following chemical equation: Web a chemical reaction is a process in which some substances change into different substances.
Types Of Chemical Reactions Worksheet Answer Key 14 Fresh New
Predict the products for, and then balance each of the following chemical reactions: Web answer key teacher notes on next page! You know that you have a balanced chemical equation when the. Web predicting products of chemical reactions. ____ nabr + ____ ca(oh)2 ___ cabr2 + ____ naoh.
16 Types Chemical Reactions Worksheets Answers /
You know that you have a balanced chemical equation when the. Chemical reactions happen when the _________ hook together. Web types of chemical reactions (*summary handout): A = red, b = blue, c = green, d = yellow 2. Web balance the following chemical equation:
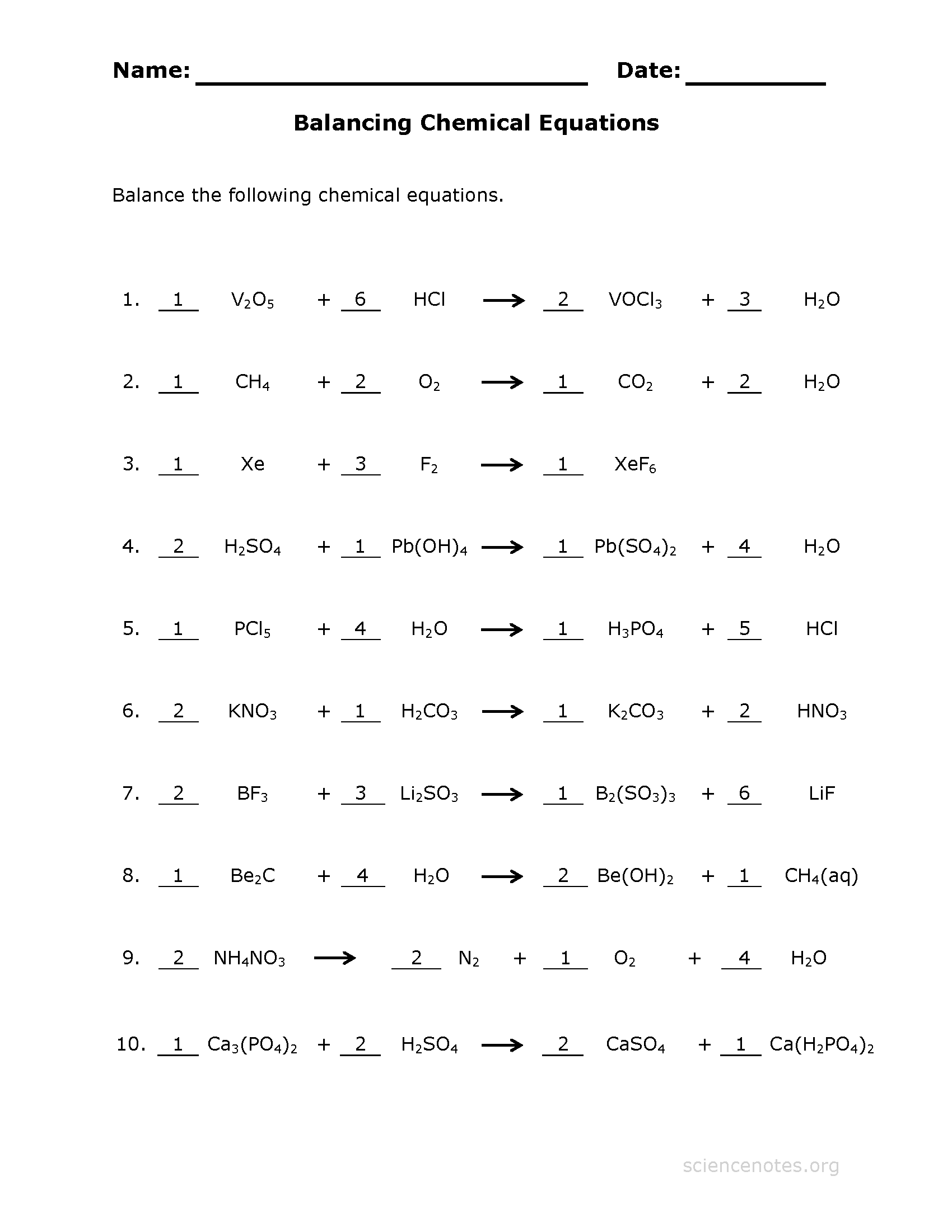
Balance Chemical Equations Worksheet 3 Answer Key Science Notes and
Write each symbol by each meaning. ____ nabr + ____ ca(oh)2 ___ cabr2 + ____ naoh. Predict the products for, and then balance each of the following chemical reactions: Web click the buttons to print each worksheet and associated answer key. 1) synthesis (slide notes) *worksheet #2:
Web classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a. Fe (no 3) 3 (aq) + h 2 s. 1) synthesis (slide notes) *worksheet #2: Balancing reactions provide the coefficients to balance the. This worksheet is designed to help you predict products. Web this worksheet set includes many detailed problems for students to complete, and also examples for each, involving.1). Chemical reactions happen when the _________ hook together. Web balance the following chemical equation: Write each symbol by each meaning. Sii4 + mg → (single replacement) 2al + 3i2 → (synthesis). Web for each reaction in part i and part ii record your observations, molecular equation, total ionic equation and net ionic equation. Web a chemical reaction is a process in which some substances change into different substances. Web types of reactions worksheet balance the following equations and indicate the type of reaction taking place: Web click the buttons to print each worksheet and associated answer key. A = red, b = blue, c = green, d = yellow 2. Web investigate and describe the occurrence of chemical reactions using the following evidence: Preparation for your unit assessment(s) ____ nabr + ____ ca(oh)2 ___ cabr2 + ____ naoh. Web predicting products of chemical reactions. Mg (oh) 2 + hcl → mgcl 2 + h 2 o.
Preparation For Your Unit Assessment(S)
Mg (oh) 2 + hcl → mgcl 2 + h 2 o. Balancing reactions provide the coefficients to balance the. Web this worksheet set includes many detailed problems for students to complete, and also examples for each, involving.1). Web types of chemical reactions (*summary handout):
A = Red, B = Blue, C = Green, D = Yellow 2.
Write each symbol by each meaning. The chemical equations in model 1 contain the phase notations (s), (l), (g), and (aq). 1) synthesis (slide notes) *worksheet #2: 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3.
Web Answer Key Teacher Notes On Next Page!
Web investigate and describe the occurrence of chemical reactions using the following evidence: Web types of reactions worksheet balance the following equations and indicate the type of reaction taking place: Web predicting products of chemical reactions. This worksheet is designed to help you predict products.
Web For Each Reaction In Part I And Part Ii Record Your Observations, Molecular Equation, Total Ionic Equation And Net Ionic Equation.
Sii4 + mg → (single replacement) 2al + 3i2 → (synthesis). Web identify the oxidizing agent, reducing agent, substance oxidized, and substance reduced in this reaction: You know that you have a balanced chemical equation when the. Web some of the worksheets for this concept are chemical reactions work answer key, identifying chemical reactions work.